Frequently Asked Questions
Everything you need to know about publishing plain language summaries
What are Plain Language Summaries of Publications articles (PLSPs)?
It is now recognised that making the results of clinical research accessible and understandable to non-specialist audiences via plain language content is vital to promote patient engagement activities and to allow the integration of new research into future patient care. Providing scientific content that is understandable to a lay audience also instils transparency in the research.
Various plain language options to accompany scientific journal publications are starting to emerge, including short-form lay abstracts and infographics as supplementary materials. Taylor & Francis has also recognized a need to build on these existing options, and improve the availability and discoverability of this extremely valuable content. To address this, we introduced a new standalone article type – the Plain Language Summary of Publication (PLSP) in 2020.
PLSPs use non-technical language to provide a summary of a recently published research article to be read and understood by non-specialists (from patients and their caregivers to healthcare professionals and decision-makers). They can have a range of authorship, but are generally written by at least one of the authors from the original publication and we also encourage the inclusion of patient authors. A patient author can be someone who has directly participated in the study, a person who lives with or is affected by a disease (such as caregivers or family members), or someone who has provided a patient perspective on a subject.
As standalone articles, PLSPs have numerous benefits.
- The high-quality of the information can be under-pinned by independent peer review. We have an Advisory Panel of patients, patient advocates and other PLS specialists who provide robust feedback on every PLSP we publish.
- The PLSP has its own unique DOI number, as with any traditional article, and can be cited in its own right by those that want to – something that isn’t as clear to do when the PLS is a supplementary file.
- More importantly, this also means that PLSPs receive their own specific article metrics, including download and citation numbers, and online impact (e.g., on social media) – allowing the authors to track the attention they receive.
- As standalone articles, PLSPs are also much more easily discoverable. They can be found using the usual publisher search tools and indexing sites, such as PubMed and Google Scholar. Crucially, they can also be found on Google, making them more discoverable by patients and the lay public.
- Finally, as with a traditional article, all the usual publishing ethics practices apply, so for example PLSPs can be corrected using the usual journal mechanisms, such as correction notices – improving transparency and ethical compliance of this type of content.
What is the difference between a PLSP and a plain language summary?
Different publishers and organizations can use different names when talking about plain language content. In T&F journals, a ‘Plain language summary’ is a short paragraph summarizing the main points of an article, which is included within the article alongside the regular scientific abstract and of a similar length (no more than 250 words). Previously these were published in our journals as a ‘Lay abstract’.
PLSPs are a longer type of PLS, providing a complete summary of a publication written in non-technical language laid out in an infographic style with images and illustrations. They can be published in two ways with T&F – alongside an article, as part of its supplementary material files, or as a standalone article in its own right. Standalone PLSPs can summarize an T&F article, or any article (or articles) from another publisher.
What types of article can be summarised in a PLSP?
Our Editors welcome PLSPs of any publication from any publisher, including original research articles and reviews.
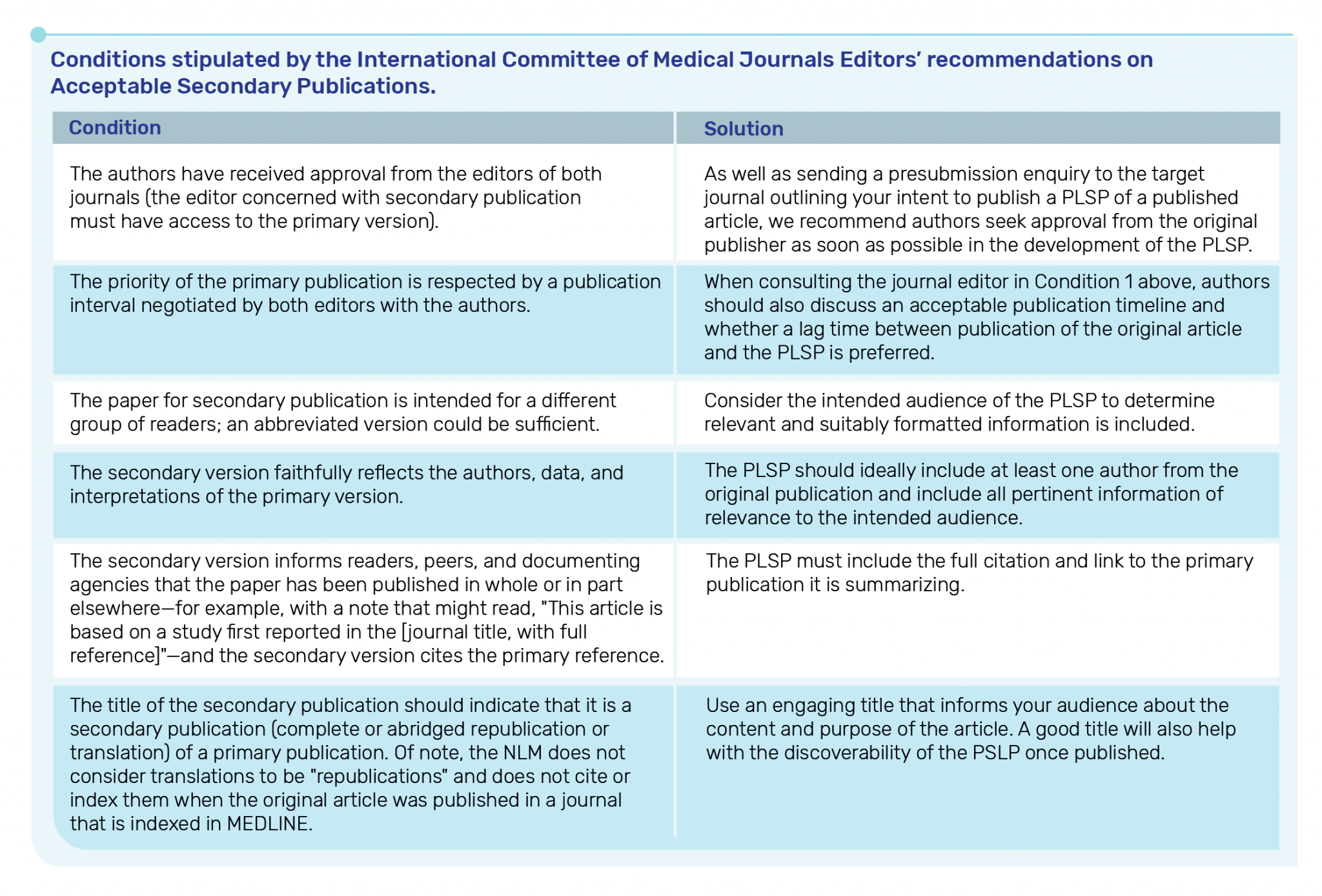
What are the ICMJE recommendations on publishing a PLSP?
T&F follows the recommendations of the ICMJE and would consider a PLSP as an Acceptable Secondary Publication (i.e., we would not consider PLSPs as duplicate publications). This is on the basis that all of the conditions stipulated by ICMJE are met. However, please note that the ICMJE recommendations are interpretable by each Publisher individually.
More details can be found here and are summarised below.
What types of article can be summarised in a PLSP?
Our Editors welcome PLSP of any publication from any publisher, including original research articles and reviews.
Are there any copyright considerations when preparing a PSLP of an article originally published in another journal?
Please refer to the section ‘What are the ICMJE recommendations on publishing PLSP?’ above.
Can a PLSP summarize more than one original article?
While PLSP generally summarize just one original publication, in some cases it might be helpful to summarize a group of related publications (perhaps relating to a cluster of trials on the same treatment), and we would be happy to discuss this further should you be interested in developing a PLSP of this kind.
Does an author from the original publication have to be an author on the PLSP?
We would encourage that an author from the original publication, or someone involved in the publication steering committee, is involved in the writing of the PLSP where possible and appropriate, but this is not compulsory. Ideally, at the least, an original author will review the content of the PLSP before submission, to check the information has been conveyed appropriately.
All authors of the PLSP must meet the authorship criteria stipulated by GPP3/ICMJE.
Can other authors who are not an author on the original publication be an author on the PLSP?
Yes – additional authors, not involved with the original publication, can be included in a PLSP. Ideally, the author group should also include a patient, such as a patient advocate or patient involved in the clinical trial, although this is not mandatory. Any author should meet the criteria stipulated by GPP3/ICMJE (authors are encouraged to refer to this tool, which highlights how each of the four criteria above can be interpreted from the patient author perspective).
Are patient authors reimbursed or paid for their time involved with being an author?
We do not pay any author to write or submit any article to a Taylor & Francis journal. Sponsors who fund the development, writing and submission of a PLSP to one of our journals may choose to reimburse a patient author however this is at their discretion and according to their own policies.
Where can I find resources on writing PLS?
We have created a set of Author Guidelines for the creation of PLSPs, which includes a list of handy resources, as well as a suggested article template and a checklist of actions when planning & writing a PLSP. Please get in touch, and we’d be happy to send you a copy!
What is the recommended reading level for PLSPs?
As stipulated in the EU Clinical Trials Regulation ‘Summaries of Clinical Trial Results for Laypersons’, PLSPs should ideally have a reading age of 12 years and above. Prior to submission, we suggest the PLSP undergoes a readability score evaluation, for instance using the Flesch-Kincaid Readability Test available at https://goodcalculators.com/flesch-kincaid-calculator/. The recommended reading ease score is 60 or more.
Can I use figures and images in a PLSP?
Yes – and we strongly encourage it, particularly original images and infographics that have been developed specifically for the PLSP. Complicated numbers, graphs, tables and data can often be more clearly understood with an illustration.
For standalone PLSPs, if you do wish to include a figure from the original scientific publication please make sure you obtain any permissions needed from the original publisher.
Who does the layout and design of PLSPs?
We have an in-house graphics team who undertake the full design and layout of PLSPs using our bespoke template. Where authors make suggestions for layout as well as graphics and imagery, we aim to incorporate these elements into the final designed PLSP, in accordance with our own house style.
We recognize that many authors have access to design services and are happy to publish already designed PLSPs. However, these must be created in InDesign and designed in accordance with the style of previously published PLSPs in the journal (please see the Publications page for examples). Template InDesign files are available upon request. Authors submitting a pre-designed PLSP must also submit the original InDesign files. Following acceptance, our in-house graphics team may edit the PLSP to align any design/imagery to our house style and ensure all relevant journal information is included within the article.
Can I include other media, such as a video, in a PLSP?
Yes – do let us know if you’d be interested in including video or audio content and we’d be happy to discuss this. Such files can be linked to from the typeset article. All media will undergo editorial review.

Can the PLSP link to ClinicalTrials.gov?
Where PLSPs summarize the publication of the data from a new clinical trial, they can and should include a link to the trial’s entry on ClinicalTrials.gov, if it has one. PLSPs should also include a link to the publication(s) it is summarizing, and any other useful resources.
Are PLSPs peer reviewed?
Yes – whether the PLS is included within an article, or is standalone, it will be externally peer reviewed to ensure that it is both an accurate reflection of the scientific article it is summarizing, and also that it is understandable to a non-specialist audience (for example, any unnecessary technical words have either been replaced or suitably explained).
All PLSPs are sent out for external review by experts in plain language content as well as patients and patient advocates. As a result, reviewers for PLSPs are reimbursed for their time via an honorarium. T&F is particularly keen to encourage patient reviewers for PLSP. If you would be interested in acting as a reviewer for our PLSPs, please get in touch!
Why are PLSPs published under a Creative Commons CC-BY-NC-ND license and not a CC-BY license?
Our aim with standalone PLSP articles is that they are freely available to all those who want to read them. However, we also believe it is important that the PLSP is read as a whole. By preventing the creation of derivatives or adaptations, this license type ensures the information presented is not altered or taken out of context. We are happy to discuss the publication of PLSP under alternative Creative Commons open licenses should this be required.
What are the fees to publish a PLSP?
The fees to publish a PLSP in any Taylor & Francis journal is $5,500. These fees cover the following:
• Publishing the PLSP open access under a CC BY-NC-ND license
• Editorial review of the PLSP prior to publication, including internal review, external peer review by patient/lay/plain language experts and editorial feedback (in terms of content, readability and design)
• In-house processing of the PLSP from submission to publication
• Full design of the final article, including creation of additional imagery, re-styling of graphics and layout into a patient-friendly format
• Online hosting of the PLSP with keywords and other tools to enhance discoverability on our journal website and associated Plain Language Summaries website
• Dissemination across social media (Twitter, LinkedIn and Facebook) using relevant hashtags and mentions
• Indexing on relevant database, such as Medline, where applicable (in accordance with the journal’s indexing status)
• Liaison with relevant patient organization to ensure they are aware of the PLSP as a tool to educate and inform their members
Why do you charge fees?
All PLSP are published on an open access basis so they are freely accessible to all wanting to understand the latest research. Therefore fees are charged to cover the costs associated with publishing the PLSP.
Where are PLSPs indexed?
Indexing of a PLSP will follow that of the regular journal content. When PLSPs are published, they are disseminated via social media and patient advocacy groups, ensuring patients can readily discover the article. Therefore we believe that traditional indexing databases, such as Medline, as well as impact factors, are not entirely relevant when publishing PLSPs as patients/lay audiences use other means to discover such articles.
How are PLSPs indexed?
As PLSPs are published like any standard journal article, they have their own DOI (Digital Object Identifier) and citation. The PLSP links to the original article in PubMed as we ensure the PLSP is indexed as a ‘comment’ on the original publication. For instance see https://pubmed.ncbi.nlm.nih.gov/33554636/. The PLSP is also discoverable alongside the ClinicalTrials.gov record – for an example see https://clinicaltrials.gov/ct2/show/NCT02200614
Are PLSPs discoverable on Google?
PLSPs are published to ensure they are discoverable across multiple sources. We recognise that readers may seek information on Google and optimise the discoverability of PLSPs on this platform. We do this by asking authors to include the words ‘plain language summary’ in their title, including key words and listing the PLSP on multiple sources, such as this website, in addition to the journal website.
Do the original articles link to the PLSP?
All PLSPs we publish must include the full citation and link to the original publication it is summarizing. Where the original publication is published in a FSG journal, a link appears alongside the original article directing readers to the PLSP (and vice versa). Where the original article is from a journal outside of our group, it is at the discretion of that journal to link to the PLSP.
The PLSP and original publication link to each other in PubMed, where we ensure the PLSP is indexed as a ‘comment’ on the original publication.
What external resources are available to learn more about PLS?
Several external resources are available that provide guidance on the development and publication of PLS as follows:
• Patient Focused Medicine Development (PFMD) ‘How-To Guide for the development and dissemination of plain language summaries of peer-reviewed publications and conference presentations’. Available to download here.
• Open Pharma recommendations for plain language summaries of peer-reviewed medical journal publications. Available to read here.
• ISMPP article ‘Plain language summaries of publications of company-sponsored medical research: what key questions do we need to address?’ Available to read here.
Are PLSPs available in other languages?
We are keen to ensure that accessibility of PLSPs is as broad as possible. Therefore, we can host translated versions of PLSPs alongside the original English language article online. The translation is available as a PDF, which can be accessed via a thumbnail on the article page. Multiple translations can be featured.
The translations can be provided by the author or we work with several partners and can provide full translation services if needed. If this is of interest, please get in touch.
How long does it take from submission to publication of PLSPs?
Standard publication of PLSPs usually occurs within 12–14 weeks of first submission. This takes into account time for peer-review, author revisions, final layout and proof approval. Should author require publication by a certain time, we can endeavor to meet this deadline where feasible.
All our journals also offer an Accelerated Publication option, our fast track publication service that provides online publication within 7 weeks of first draft submission.
