- Written by Rachel Jenkins
ESMO 2023 round-up: addressing clinical trial disparities and solutions
The European Society for Medical Oncology (ESMO) Congress 2023 (20-24 October; Madrid, Spain) brought together an estimated 33,000 individuals from across the globe. Excitingly, we saw many plain language summary (PLS) abstracts featured in posters throughout the event, which we hope will become more commonplace and aid with readability. This year’s congress, notably shone a spotlight on the issues and areas for improvement seen in clinical trials, ranging from the implementation of digital solutions in countries such as Spain and Italy and the inclusion of underrepresented groups in trials. Read more about these topics in our ESMO 2023 round-up below!
How digital solutions reveal clinical trial disparities in Spain.
Many eligible patients in Spain do not participate in clinical trials (studies) due to the lack of reliable information about trial availability. Researchers explored this issue by analyzing the data from public registries (such as clinicaltrial.gov), the Spanish Ministry of Health and an AI-based clinical trial search engine called Trialing – a digital resource where doctors can directly refer their patients to available trial sites.
A total of 561 Spanish oncology centers participated in this study, of which 23% offered clinical trials. These centers were categorized as either ‘small’, ‘medium’, ‘large’ or ‘giant’, depending on how many clinical trials they offered. A center was considered ‘small’ if it offered less than 20 trials, ‘medium’ if it offered between 21 and 75, ‘large’ if it offered between 76 and 150 and ‘giant’ if it offered more than 150 clinical trials.
The researchers discovered the following:
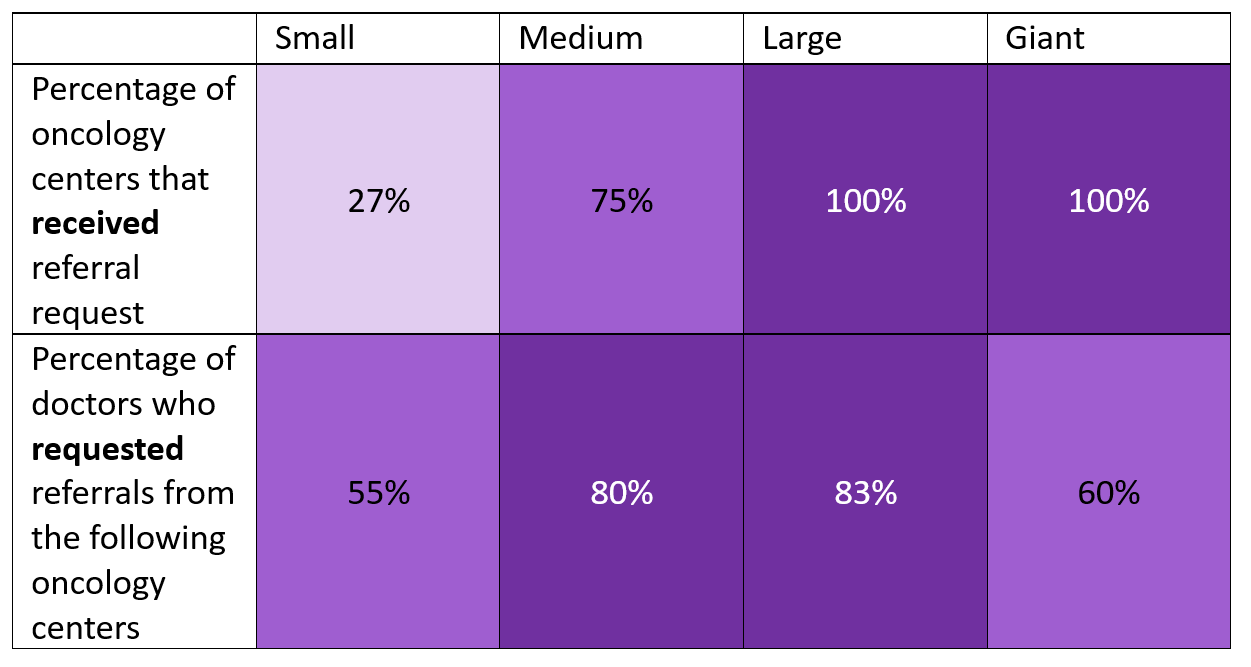
When exploring how geographically dispersed the clinical trials were, researchers found that most communities in Spain have no more than 10% of the trials available. Only 2 of the 17 administrative regions in Spain account for more than 50% of the available clinical trials, this demonstrates that the distribution of clinical trials in Spain is highly concentrated.
Source: Hardy-Werbin M, Mosquera JJG, Gallardo SG et al. 1716P Addressing clinical trial disparities in Spain: A digital solution. Presented at ESMO 2023 (20-24 October, Madrid, Spain).
Are a sufficient number of ethnic minorities recruited for trials studying gynecological cancer treatments? ESMO 2023 round-up
Pharmacogenomics (the study of how genes affect an individual’s treatment response) shows that treatment outcomes may differ between ethnic groups; diverse populations must be included in clinical trials to ensure that improvements in healthcare benefit all individuals rather than causing an increase in health disparities. A review was conducted to look at the participation of ethnic minority groups in the clinical trials of systemic anti-cancer therapies (any drug treatment used to control or treat cancer) for gynecological cancers.
The researchers analyzed several databases including Medline, Embase and ClinicalTrials.gov and found 26 clinical trials that met the inclusion criteria of the study; this amounted to 17,041 participants in total. They found that 79.8% were Caucasian, 9.1% Asian, 3.7% Black/African American, and 6.1% Other/Unknown.
Though Caucasian participants were over-represented in clinical trials across disease indications, they were recruited at a higher rate in phase II trials compared to phase III trials. Black/African American participants were also recruited at a higher rate in phase II trials compared to phase III trials.
The global distribution of research sites was also assessed. Of the 26 studies analyzed, the majority, 20 were conducted in multiple countries. Of the 5478 sites where the trials were conducted, 80.1% were located in North America or Europe, 3.4% in East Asia, and 16.5% in other regions. Notably, no sites were located in Africa or South Asia.
This review highlights that ethnic minorities are under-represented in gynecological clinical trials, which may reflect clinical trials for similar pathologies.
Source: Steventon L, Man K, Chambers P, Nicum S & Wei L. 817P A systematic review of recruitment of ethnic minorities to RCTs of systemic anti-cancer therapies in gynaecological cancers. Presented at ESMO 2023 (20-24 October, Madrid, Spain).
Can decentralized clinical trials be effectively implemented in Italy?
Decentralized clinical trials (DCTs) are trials that are conducted at different locations other than a traditional clinical trial site, this allows participants to join the trials remotely using digital health technologies. Though implementing DCTs was a big priority during the COVID-19 pandemic there are still some points of concern that need to be addressed.
In June of last year, a company called Life Science Innovation shared an anonymous online Survey with the Italian Group of Data Manager; the aim was to look at how widespread DCT elements were in Italian clinical research centers. The survey was completed by 35 study coordinators, most (54.3%) of whom were from public hospitals.
Despite DCTs becoming a dramatic priority during the pandemic, 60% of respondents did not have any experience in conducting them, though the majority (72.7%) expressed an interest in DCTs for the future. Those who weren’t interested in DCTs (50%) may be less keen due to the specific skill set that is required to conduct a DCT.
As digital solutions are closely tied to DCTs, the survey analyzed the answers to specific digital solutions. Most respondents used devices (such as sensors) for remote parameter collection (60%). The majority of respondents also used tools for collecting patients’ data such as apps for monitoring therapeutic adherence (60%) and for delivering Electronic Patient Reported Outcomes (60%).
The survey also used the following scaling system to ask more questions.
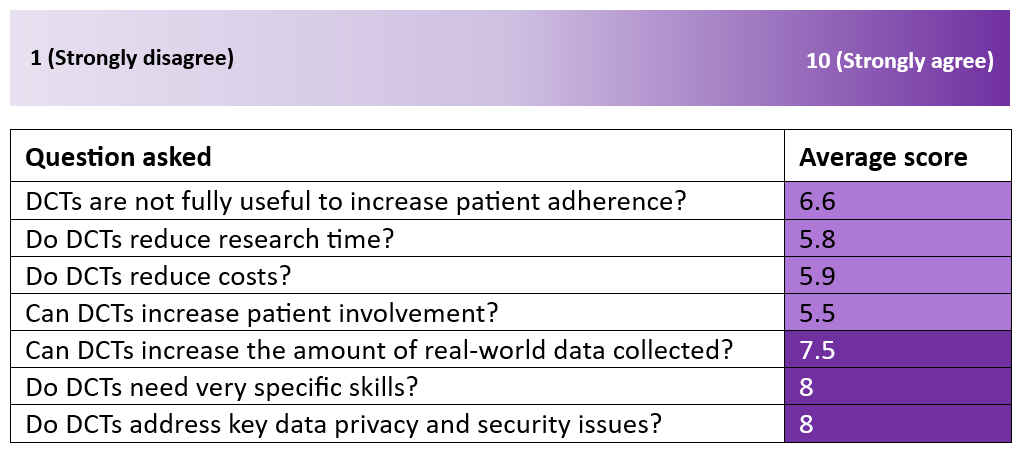
This survey shows that the interest in implementing studies with some digital components remains limited and these crucial aspects regarding Italy’s ability to implement DCTs in the short run should be addressed.
Source: Cagnazzo C, Mannozzi F, Stabile S et al. 1719P Decentralized clinical trials: Is there a space in Italy? Presented at ESMO 2023 (20-24 October, Madrid, Spain).
Access the poster presentations from ESMO 2023 by visiting www.cslide.ctimeetingtech.com/esmo2023/attendee/confcal_2.
Interested in more conference content like this ESMO 2023 round-up? Take a look at our ASCO 2023 round-up by visiting www.plainlanguagesummaries.com/asco-2023-roundup-patient-centred-communication/.
This post was authored by Olivia Alexander (Editor – Plain Language Summaries; Future Science Group).
